Any comments, suggestions or just looking for a chat about this subject? Don't hesitate and leave a comment on our improved comment section down below the article!
By David Cole-Hamilton - Emeritus Professor of Chemistry, University of St Andrews
Period pains. - Image Credit: European Chemical Society
It is amazing to think that everything around us is made up from just 90 building blocks – the naturally occurring chemical elements. Dmitri Mendeleev put the 63 of these known at the time into order and published his first version of what we now recognise as the periodic table in 1869. In that year, the American civil war was just over, Germany was about to be unified, Tolstoy published War and Peace, and the Suez Canal was opened.
There are now 118 known elements but only 90 that occur in nature. The rest are mostly super-heavy substances that have been created in laboratories in recent decades through nuclear reactions, and rapidly decay into one or more of the natural elements.
Where each of these natural elements sits in the periodic table allows us to know immediately a great deal about how it will behave. To commemorate the 150th anniversary of this amazing resource, UNESCO has proclaimed 2019 as the International Year of the Periodic Table.
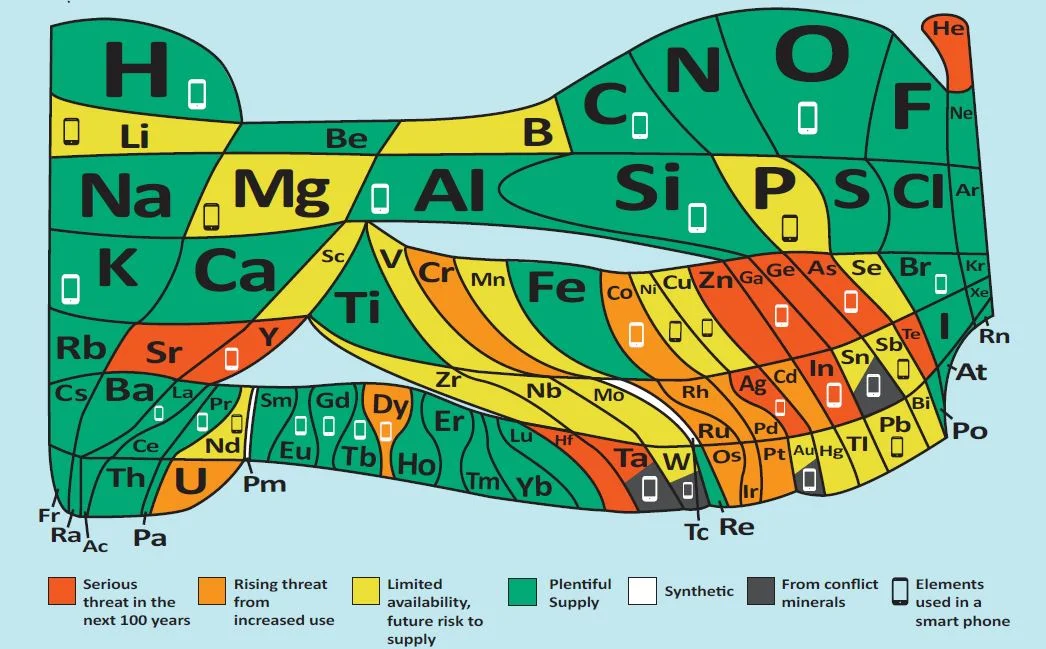
As part of the celebrations, the European Chemical Society has published a completely new version of the periodic table – see main image. It is designed to give an eye-catching message about sustainable development; based on an original idea in the 1970s from the American chemist William Sheehan, the table has been completely redrawn so that the area occupied by each element represents its abundance on a log scale.
Red for danger
Each area of the new table has been colour coded to indicate its vulnerability. In most cases, elements are not lost but, as we use them, they become dissipated and much less easy to recover. Red indicates that dissipation will make the elements much less readily available in 100 years or less – that’s helium (He), silver (Ag), tellurium (Te), gallium (Ga), germanium (Ge), strontium (Sr), yttrium (Y), zinc (Zn), indium (In), arsenic (As), hafnium (Hf) and tantalum (Ta).
To give just a couple of examples, helium is used to cool the magnets in MRI scanners and to dilute oxygen for deep sea diving. Vital rods in nuclear reactors use hafnium. Strontium salts are added to fireworks and flares to produce vivid red colours. Yttrium is a component of camera lenses to make them shock and heat resistant. It is also used in lasers and alloys. Gallium, meanwhile, is used to make very high-quality mirrors, light-emitting diodes and solar cells.
From strontium with love. - Image Credit: Sharosh Rajasekher via Unsplash
Meanwhile, the orange and yellow areas on the new periodic table anticipate problems caused by increased use of these elements, too. Green means that plenty is available – including the likes of oxygen (O), hydrogen (H), aluminium (Al) and calcium (Ca).
Read more: The periodic table is 150 – but it could have looked very different
Four elements – tin (Sn), tantalum (Ta) tungsten (W) and gold (Au) – are coloured in black because they often come from conflict minerals; that is, from mines where wars are fought over their ownership. They can all be more ethically sourced, so it’s intended as a reminder that manufacturers must carefully trace their origin to be sure that people did not die in order to provide the minerals in question.
Smartphone shortages
Out of the 90 elements, 31 carry a smartphone symbol – reflecting the fact that they are all contained in these devices. This includes all four of the elements from conflict minerals and another six with projected useful lifetimes of less than 100 years.
Let us consider indium (In), for instance, which is coloured red on the table. Every touch screen contains a transparent conducting layer of indium tin oxide. There is quite a lot of indium, but it is already highly dispersed. It is a byproduct of zinc manufacture, but there is only enough from that source for about 20 years. Then the price will start to rise quickly – unless we do something to preserve current stocks.
The three main possibilities are: replace, recycle or use less. Huge efforts are being made to find alternative materials based on Earth-abundant elements. Reclaiming indium from used screens is possible and being attempted. But when we look at the Periodic Table and the very precious nature of so many of the elements, can we possibly justify changing our phone every two or so years?
Image Credit: Freestocks via Unsplash
At present over 1m phones are traded in every month in the UK alone (10m in Europe, 12m in the US). When we trade in our smartphones, many of them go to the developing world initially for reuse. Most end up in landfill sites or attempts are made to extract a few of the elements under appalling conditions. The other elements remain in acidic brews. This, and the very many that lie around in drawers, is how the elements in mobile phones become dissipated.
The number of phones we trade in could be greatly reduced and with it the demand on limited resources such as indium. In this context, the recent Apple profit warning, partly due to customers replacing their iPhones slightly less frequently, was at least a sign of improvement.
But as the new version of the periodic table underlines, we must do all we can to conserve and recycle the 90 precious building blocks that make up our wonderfully diverse world. If we don’t start taking these problems more seriously, many of the objects and technologies that we now take for granted may be relics of a more abundant age a few generations from now – or available only to richer people.
Source: The Conversation
If you enjoy our selection of content please consider following Universal-Sci on social media: