Smartphones and other electronics have improved vastly over the past 10 years - Image Credit: freestocks via Unsplash - HDR tune by Universal-Sci
In modern times we tend to use our electronic devices more intensively than before. Smartphones and tablets are getting more substantial screens, better processors, more ram, more storage and more powerful GPUs. In contrast with these vast quality improvements, battery life seems to stay behind significantly. The batteries of today are not that much better than they were 10 years ago, while the difference between a 2019 smartphone and a 2009 smartphone is like night and day.
The problem with improving contemporary batteries is that in order to store a lot of energy, you have to use heavier materials to replace the currently used graphite. As it is now, the battery life of, for example, your phone is dependent on the number of lithium ions it can store in the battery's negative electrode material. If it is out of ions, it won't be able to create an electrical current anymore.
Read more: Why does smartphone battery life get worse and worse as it gets older?
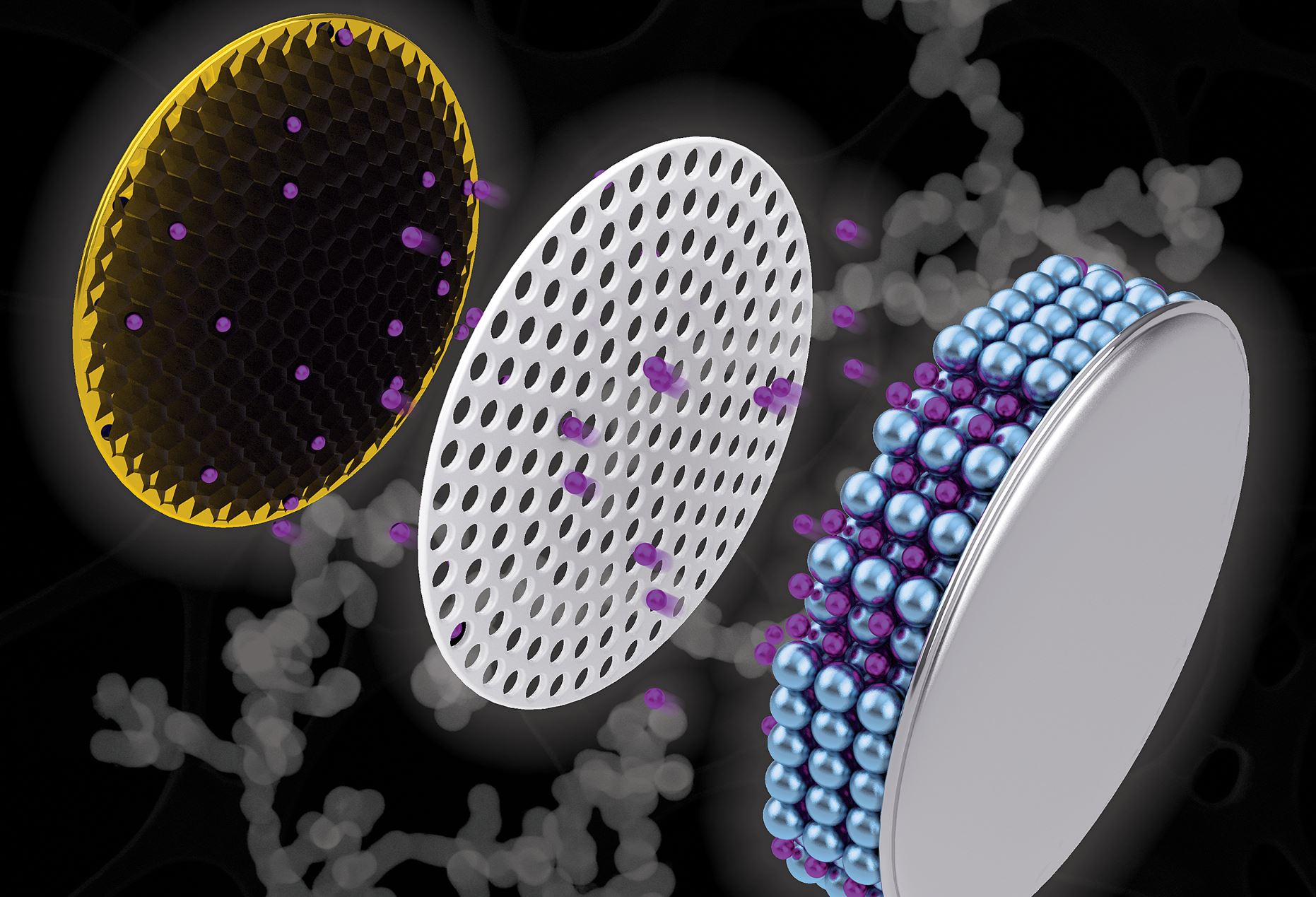
Researchers from Purdue University may have found a new method in which potential replacement materials could be restructured into a new electrode composition. It is expected that this new design will increase the lifespan of batteries as well as decrease their charge time. The scientists created a net-like construction made from antimony, a substance that enhances lithium-ion charge capacity in batteries. This composition is called a nanochain.
The scientists then compared the nanochain electrodes with graphite electrodes and found that when coin cell batteries with the nanochain electrode were only charged for half an hour, they attained double the lithium-ion capacity and kept it for at least a hundred charge-discharge cycles.
An artistic depiction of a coin cell battery with a copper electrode (left) containing a black nanochain structure - Image Credits: Purdue University illustration/Henry Hamann
Some types of batteries are already utilizing carbon-metal composites similar to anatomy metal negative electrodes. But most of them are used commercially, and there are some concerns with regards to safety as the materials used tend to expand a lot
Vilas Pol, an associate professor at Purdue University, stated that they want to adapt to that expansion for smartphone batteries. As it would be unsafe for people to carrying around in their pockets otherwise. The researchers are trying to adjust for this expansion by applying a reducing agent and a nucleating agent to connect the tiny antimony particles into a nanochain shape.
P. V. Ramachandran, professor of organic chemistry, stated that their method of making nanoparticles reliably provides the chain structures. It turns out that the nanochain keeps the ion capacity stable for at least 100 charging / discharging cycles. The researchers observed practically no change between the first cycle and the 100th cycle. They have therefore no reason to believe that cycles after the 100th cycle will be different.
All in all, with a bit of luck we might yet see some real improvements in the battery performance of our electronics in the future.
Sources and further reading: Three-Dimensional Antimony Nanochains for Lithium-Ion Storage / Purdue press release / Lithium-ion battery
If you enjoy our selection of content please consider following Universal-Sci on social media: